Refuge chambers should be sealed environments to protect people from outside contaminants. So, how do they remove carbon dioxide (CO2) and carbon monoxide (CO) from an airtight environment?
Chemical scrubbers, as the name suggests remove ‘scrub’ the air by using a chemical to remove harmful gases. Refuge chambers use dry chemical scrubbers to remove CO and CO2 from the internal environment, especially if compressed air fails or becomes compromised.
Providing breathable air within a refuge chamber is a challenge, along with disposing of the carbon dioxide and carbon monoxide we breathe. As occupants within the sealed environments breathe, CO2 and CO will build up. If these gases are not removed from the internal atmosphere, they can build to toxic levels and poison the chambers inhabitants.
Chemical scrubbers must efficiently handle the dangerous build-up because the air quality within a refuge chamber must be maintained,
How to Remove Carbon Dioxide from a Refuge Chamber
Carbon Dioxide Scrubber Chemical
Traditionally, chemical scrubbers use a type of soda-lime to lock onto CO2 molecules. Soda-lime is a mixture of NaOH & CaO chemicals, made by treating slaked lime with concentrated sodium hydroxide solution. Commonly, it is used in granular form in closed breathing environments, such as submarines, recompression chambers, and safe havens, to remove carbon dioxide from the air to prevent CO2 retention and carbon dioxide poisoning.
The overall balanced equation is:
H2O/ NaOH
CO2 + Ca(OH)2 → CaCO3 + H2O + heat (in the presence of water)
Access More Resources
How to Remove Carbon Monoxide from a Refuge Bay
Carbon Monoxide Scrubber Chemical
Carbon monoxide is removed via catalytic oxidation, producing less harmful CO2. The reaction mass is made from manganese dioxide and copper oxide.
The overall balanced equation is:
CO + O2 = CO2

Access More Resources
How Do Chemical Scrubbers Work?
Chemical scrubbing is the process of removing undesired gases from the air using chemical reactions that change the composition of the gas to water and heat as they pass through the system.
Scrubbing mechanisms work in conjunction with active chemicals to ‘scrub’ the build-up of harmful CO and CO2 from the air inside the refuge chamber. Firstly, chemical scrubbers draw air in the from the atmosphere with the assistance of an internal fan. Then, the air travels through the scrubbing material, where the chemical reaction occurs. Finally, the processed air is returned to the internal atmosphere.
The design and chemical distribution will impact the efficacy of a refuge chambers internal scrubber. Two crucial elements are the scrubber materials and their distribution.
Pressure and Flow Distribution
The distribution of air pressure and flow impacts the effectiveness of the chemical reaction. Therefore, plenum trays are used within a scrubber to evenly distribute the load and circulation of air within the machine.
Uneven distribution and force can result in high-pressure pockets or defects in the chemical bed. These weaknesses allow air to flow through the path of least resistance, reducing the filtration ability of the chemical materials – impacting duration. In some cases, this reduction can be as high as 60% (14.4 hours).
Chemical Scrubber Material
The size and shape of the scrubbing material itself are also significant. For instance, size and form create a packing density matrix that requires a specific flow and pressure differential to ensure it is effective.
Variants of this density will impact the removal efficiency and duration of the breathing space.
Because of its critical importance, any changes to the chemical or the equipment would require re-calculation. This is to ensure, as a life support system, it can remove CO2 and CO in the chamber over the full duration of entrapment.
The scrubber chemical is usually in two alternate shapes:
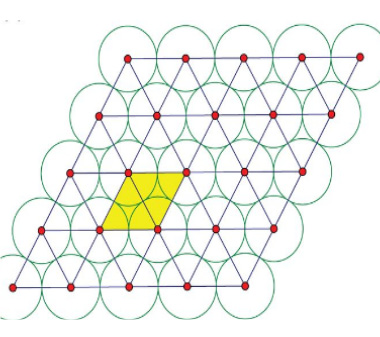
- Spherical Grain: The most commonly occurring scrubber compound is a diver-grade spherical granule.
- Crushed Triangle: Due to its shape, the triangle allows for more material to be stored within the same volume.
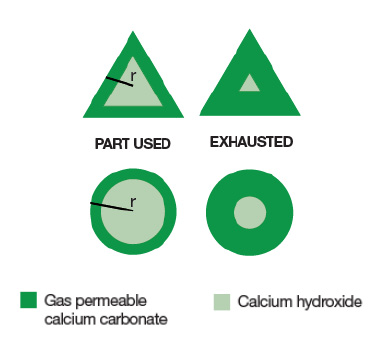
A triangle granule allows more material to be consumed before exhaustion. As the area of the triangle is less than the circle with the same wall thickness. In comparison, using circular granules reduces the amount of chemical you have available to you in that volume, which means less duration.
When packed together, the circles have more space between them than the triangles. However, triangular particles are packed down in a way that inhibits channelling – where air flows through gaps as a path of least resistance and doesn’t use the chemicals around it.